Abstract
Background: Amyloidosis can stem from various amyloid types. Accurate amyloid typing is crucial for management and prognostic purposes. Typing used to rely on immunohistochemistry and immunofluorescence methods, but is subject to considerable false positive and false negative results as well as dependence on laboratory expertise. Mass spectrometry (MS) has emerged in the past years as the gold-standard typing method with sensitivity and specificity approaching 100%. It is helpful in elimination of cases where monoclonal protein studies are an incidental finding, while amyloidosis type is in fact non-AL. With this technique in clinical practice for nearly a decade, accurate description of AL amyloidosis from a clinical and laboratory data is now feasible, which allows a refinement of the presenting features of the disease compared to prior cohort descriptions.
Methods: Five hundred and ninety two patients with biopsy-proven, MS confirmed AL amyloidosis diagnosed between 2008 to 2015 are included. Monoclonal protein studies were performed at diagnosis before treatment initiation and included: serum immunofixation (IFE), urine IFE and serum free light chain assay (sFLC). Comparison was made with data from prior study pre-MS era from our institution: (Gertz MA, Kyle RA. (1989) Primary systemic amyloidosis: a diagnostic primer, Mayo Clinic Proceedings).
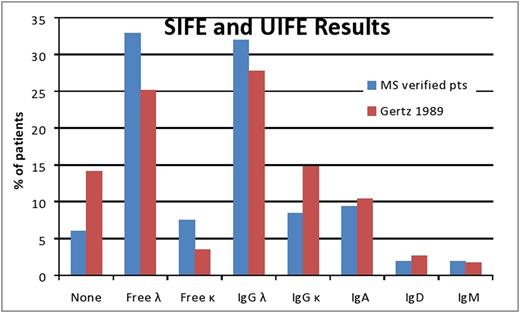
Results: A lambda clone was seen in 75% of patients in the MS-verified cohort, compared to 62% in the prior study. The rate of detection of a monoclonal protein by serum or urine IFE was 94%, as compared to 86% previously (Figure). The distribution of monoclonal proteins also differed with more patients in the earlier cohort with an IgG kappa and fewer without an intact immunoglobulin. Abnormal sFLC ratio (>1.65 or <0.26) was detected in 91% of patients in the current series, while 78% of patients had ratio >3.3 or <0.13. Overall, the median difference between involved to uninvolved light chains (dFLC) was 25 (11-65) mg/dL. Two patients had a negative dFLC (-0.7 & -5.7 mg/dL; both lambda type), while 13 patients (2%) had dFLC of less than 1 mg/dL. In the current MS-verified cohort, among the 557 patients with all monoclonal studies available (SIFE, UIFE, sFLC), only one patient (0.2%) had normal monoclonal protein studies. Greater than 10% bone marrow plasma cells were seen in 35% of patients in the MS-verified cohort versus only 25% in prior earlier cohort who had this degree of bone marrow involvement. Other differences between the two groups were male gender (64% versus 61%), frequency of cardiac involvement (76% versus 40.5%) and frequency of nerve involvement (26% versus 19%). Frequencies of renal (53% vs 48%) and liver involvement (18% vs 16%) were similar between studies.
Conclusion: When mass spectrometry is used to definitively type amyloid and state of the art serum and urine immunofixation and serum free light chain analysis are used, only a fraction of a percent of AL patients have negative monoclonal protein studies, unlike historical reports. Whether this is due to a drift in the prevalence of monoclonal proteins over time or a reflection of misclassification in an era with limited diagnostic capability will require further study.
Gertz: Millennium: Consultancy, Honoraria; Celgene, Novartis, Smith-Kline, Prothena, Ionis, Amgen: Honoraria. Dingli: Millenium: Consultancy; Alexion Pharmaceuticals: Consultancy; Janssen: Consultancy; Takeda: Consultancy; Karyopharm Therapeutics: Research Funding. Kapoor: Takeda, Celgene and Amgen: Research Funding. Russell: Vyriad: Equity Ownership; Imanis Life Sciences: Equity Ownership. Kumar: Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy; Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding; Skyline: Honoraria. Dispenzieri: Celgene, Millenium, Pfizer, Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal